Adverum Biotechnologies Presents Long-term Data through March 10, 2021 from the OPTIC Trial of ADVM-022 Intravitreal Gene Therapy in Treatment-experienced Wet AMD Patients at ARVO 2021
-- Long-term durability and maintained efficacy; sustained robust aflibercept protein expression observed --
-- 60% of patients injection free beyond 1 year following 2 x 10^11 single dose --
/EIN News/ -- REDWOOD CITY, Calif., May 01, 2021 (GLOBE NEWSWIRE) -- Adverum Biotechnologies, Inc. (Nasdaq: ADVM), a clinical-stage gene therapy company targeting unmet medical needs in ocular and rare diseases, today announced new long-term data from the OPTIC clinical trial of ADVM-022 single intravitreal (IVT) injection gene therapy in patients requiring frequent anti-VEGF injections for their neovascular or wet age-related macular degeneration (wet AMD). Safety and efficacy data from patients followed for a median of 88 and 68 weeks at the 2 x 10^11 vg/eye dose (for Cohorts 2 & 3, respectively) and 104 and 36 weeks at the 6 x 10^11 vg/eye dose (for Cohorts 1 & 4, respectively) are being presented at the Association for Research in Vision and Ophthalmology (ARVO) 2021 Virtual Meeting with a pre-recorded presentation uploaded on April 16, 2021.
“The long-term OPTIC data show the potential for ADVM-022 to offer disease modifying treatment for patients with wet AMD,” said Laurent Fischer, M.D., chief executive officer of Adverum Biotechnologies. “Patient safety is our absolute priority and following the unexpected adverse event we reported this past week in a patient treated with the 6e11 high dose in the INFINITY study in diabetic patients with macular edema, we are unmasking the INFINITY study in order to analyze all data available and monitor every patient who has received our gene therapy. We are also working closely with our data monitoring committee and scientific advisors and conducting a thorough review of all the data from our ADVM-022 program. We will report our findings as the analysis progresses to inform next steps for development.”
Dr. Fischer continued, “In OPTIC, ADVM-022 with wet AMD has demonstrated durability out to two years with the ease of a single, in-office intravitreal injection. We believe that we are well within the therapeutic window with the 2e11 dose with 60% of patients supplemental injection free beyond one year. Additionally, the aflibercept protein levels at the 2e11 dose were within the modeled therapeutic range and sustained out to at least one year, consistent with levels observed 4-6 weeks after an aflibercept injection.”
The data reported in this press release and the related statements relate only to the OPTIC clinical trial evaluating ADVM-022 gene therapy for the treatment of wet AMD.
Adverum reported new interim data from the OPTIC trial (March 10, 2021 cutoff date, n=30) that continue to demonstrate the potential of ADVM-022 to greatly reduce the anti-VEGF injection burden for patients with wet AMD:
- All ADVM-022-related ocular adverse events (AE) were mild (80%) to moderate (20%) in OPTIC patients with wet AMD. No clinical or fluorescein evidence of posterior inflammation
- No vasculitis, retinitis, choroiditis, vascular occlusions, or endophthalmitis
- Inflammation when observed was mild and responsive to steroid eye drops
- At 2 x 10^11 vg/eye dose, ocular inflammation was minimal and responsive to steroid eye drops; 87% of patients (13/151) have discontinued steroid eye drops
Cohort 3 Safety Data for 2 x 10^11 vg/eye Dose:
- A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b6f22004-1612-4316-a481-9ac63157b54f
- A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/cbe15798-cd87-444f-b81a-f989dd73fc4a
- A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/7e3ef263-ebd4-4a27-a783-b41515ed8bdd
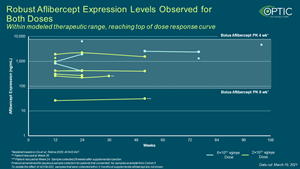
- Durable expression of aflibercept following a single, in-office IVT injection of ADVM-022, for both doses (2 x 10^11 vg/eye and 6 x 10^11 vg/eye)
- Maintained or gained vision (mean BCVA2)
- Maintained to improved retinal anatomy (mean CRT3)
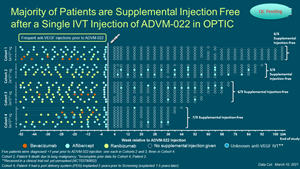
- Majority of patients are supplemental anti-VEGF injection free:
- 60% of patients (9/151) injection free following 2 x 10^11 vg/eye dose beyond one year
- 73% of patients (11/151) required zero or one injection following 2 x 10^11 vg/eye at one-year
- 87% of patients (13/154) injection free following 6 x 10^11 vg/eye dose
- A photo accompanying this announcement is available at: https://www.globenewswire.com/NewsRoom/AttachmentNg/f20337ce-b5a4-45de-8895-404bcd79b843
- Substantial reduction in annualized anti-VEGF injection frequency5 following ADVM-022 in patients who previously required frequent injections:
- 85% reduction for 2 x 10^11 vg/eye
- 96% reduction for 6 x 10^11 vg/eye
- Robust sustained aflibercept expression levels within therapeutic range was observed for both doses and reaching the top of the dose response curve.
- A photo accompanying this announcement is available at: https://www.globenewswire.com/NewsRoom/AttachmentNg/e83dc5f6-25da-4922-96f3-49e30b1c7811
- A photo accompanying this announcement is available at: https://www.globenewswire.com/NewsRoom/AttachmentNg/e83dc5f6-25da-4922-96f3-49e30b1c7811
OPTIC Clinical Trial Data:
| Results Following a Single ADVM-022 Dose: |
Cohort 1 |
Cohort 2 |
Cohort 3 | Cohort 4 | |||
| Patients | n=6 | n=6 | n=9 | n=9 | |||
| Median (Range) Follow-up Visit (Weeks) |
104 (All) |
88 (646 to 92) |
68 (48 to 72) |
36 (32 to 44) |
|||
|
ADVM-022 Dose |
High dose 6 x 10^11 vg/eye |
Low dose 2 x 10^11 vg/eye |
Low dose 2 x 10^11 vg/eye |
High dose 6 x 10^11 vg/eye |
|||
|
Prophylactic Steroid Regimen |
13-day oral | 13-day oral | 6-week eye drops | 6-week eye drops | |||
| Supplemental Anti-VEGF Injection Use: | |||||||
| Number of patients supplemental injection free | 6/6 patients | 3/6 patients | 6/9 patients | 7/9 patients | |||
| Follow-up BCVA2 and CRT3: | |||||||
| All Patients | All Patients | Supp. IVT-free Patients 50% (3/6) |
All Patients |
Supp. IVT-free Patients 67% (6/9) |
All Patients | Supp. IVT-free Patients 78% (7/9) |
|
| BCVA mean change from baseline (letters) | -1.3 | -1.5 | -1.0 | +1.4 | +4.3 | -0.2 | -0.4 |
| CRT mean change from baseline (μm) | -8.7 μm | -28.2 μm | -30.3 μm | -134.4 μm | -181.7 μm | -77.1 μm | -77.3 μm |
| 1 | All patients from Cohort 2 (n=6) and Cohort 3 (n=9) |
| 2 | Best corrected visual acuity (BCVA) |
| 3 | Central retinal thickness (CRT) |
| 4 | All patients from Cohort 1 (n=6) and Cohort 4 (n=9) |
| 5 | Annualized rate (Before) = (number of IVTs in 12 months prior to ADVM-022) / (days from the first IVT in the past 12 months to ADVM-022 / 365.25) Annualized rate (After) = (number of aflibercept IVTs since ADVM-022) / (days from ADVM-022 to the last study follow-up / 365.25) |
| 6 | A patient missed visits after week 64 due to worsening of COPD and died of a severe pneumonia due to lung malignancy at ~76 weeks |
Brandon Busbee, M.D., partner, Tennessee Retina Physicians, and investigator in OPTIC, said, “I appreciate Adverum putting patient safety first as they seek to thoroughly review the data from the ADVM-022 program. I look forward to partnering with the company on ADVM-022’s future development for patients with wet AMD.”
ARVO 2021 Presentations
Presentation Title: Phase 1 Study of Intravitreal Gene Therapy with ADVM-022 for Neovascular AMD (OPTIC Trial)
Session: AMD: Clinical research - New Therapies and Technologies
Date and Time: May 3, 2021 from 4:30 PM to 6:00 PM ET
Presenter: Brandon G. Busbee, M.D., partner, Tennessee Retina Physicians
Poster Title: Preclinical Evaluation of ADVM-062, a Novel Intravitreal Gene Therapy for the Treatment of Blue Cone Monochromacy
ADVM-062 is a one-time intravitreal (IVT) gene therapy utilizing AAV.7m8 to provide cone-specific expression of human L-opsin.
Session: Drug Delivery and Gene Therapy
Date and Time: May 3, 2021 from 11:15 AM to 1:00 PM ET
Presenter: Ruslan Grishanin, director translational science, Adverum Biotechnologies
These data presentations are available to ARVO participants and are posted on the Publications section of the Adverum’s website. Adverum is focused on conducting a thorough review of data from the ADVM-022 program in the context of the recent unexpected adverse event in the ADVM-022 INFINITY DME study and is therefore canceling its webcast to review these new OPTIC data, which was previously scheduled for Sunday, May 2, 2021.
About the OPTIC Phase 1 Trial of ADVM-022 in Wet AMD
This multi-center, open-label, dose-ranging trial is designed to assess the safety and tolerability of a single intravitreal (IVT) administration of ADVM-022 in patients with wet AMD. Patients in OPTIC are difficult-to-treat and had previously received frequent anti-vascular endothelial growth factor (VEGF) treatment. Patients received a 6 x 10^11 vg/eye of ADVM-022 in Cohort 1 (n=6) and Cohort 4 (n=9) and patients received a 2 x 10^11 vg/eye of ADVM-022 in Cohort 2 (n=6) and Cohort 3 (n=9). Patients in Cohorts 3 and 4 received six weeks of prophylactic steroid eye drops rather than 13 days of prophylactic oral steroids which were used in Cohorts 1 and 2. The primary endpoint of the trial is the safety and tolerability of ADVM-022 after a single IVT administration. Secondary endpoints include changes in best-corrected visual acuity (BCVA), measurement of central retinal thickness (CRT), as well as the need for supplemental anti-VEGF injections. Each patient enrolled will be followed for a total of two years.
For more information, please visit https://clinicaltrials.gov/ct2/show/NCT03748784.
About ADVM-022 Gene Therapy
ADVM-022 utilizes Adverum’s propriety vector capsid, AAV.7m8, carrying a codon optimized aflibercept coding sequence under the control of a proprietary expression cassette. ADVM-022 is administered as a one-time intravitreal injection (IVT), designed to deliver long-term efficacy and reduce the burden of frequent anti-VEGF injections, and improve real-world vision outcomes for patients with wet age-related macular degeneration (wet AMD) and diabetic macular edema (DME).
In recognition of the need for new treatment options for wet AMD, the U.S. Food and Drug Administration granted Fast Track designation for ADVM-022 for the treatment of wet AMD.
About Wet AMD
Age-related macular degeneration (AMD) is a progressive disease affecting the macula, the region of the retina at the back of the eye responsible for central vision. In patients with wet AMD, an aggressive form of AMD, abnormal blood vessels grow underneath and into the retina. These abnormal blood vessels leak fluid and blood into and beneath the retina, causing vision loss.
Wet AMD is a leading cause of vision loss in patients over 60 years of age, with a prevalence of approximately 1.2 million individuals in the U.S. and 3 million worldwide1. The incidence of new cases of wet AMD in the U.S. is approximately 150,000 to 200,000 annually, and this number is expected to grow significantly as the country’s population ages2,3.
The current standard-of-care therapies for wet AMD are anti-VEGF proteins. These therapies can be burdensome, as patients generally require chronic intravitreal (IVT) injection of anti-VEGF protein every 4-12 weeks. Compliance with this regimen can be difficult for patients and their caregivers, leading to compliance deficiencies and loss of vision from underdosing. It is estimated that these standard-of-care branded anti-VEGF therapies used for the treatment of wet AMD, DR, retinal vein occlusion, and other ocular diseases generated in excess of $11 billion in sales worldwide in 20204.
1 Arch Ophthalmol. 2004;122(4):564-572. doi:10.1001/archopht.122.4.564.
2 Brown GC, Brown MM, Sharma S, et al. The Burden of Age-Related Macular Degeneration: A Value-Based Medicine Analysis. Transactions of the American Ophthalmological Society. 2005.
3 California Retina Consultants. Advances in Wet AMD. Available at: https://www.californiaretina.com/advances-in-wet-amd/
4 Year-end 2020 financial statements from Regeneron, Roche, and Novartis.
About Adverum Biotechnologies
Adverum Biotechnologies (Nasdaq: ADVM) is a clinical-stage gene therapy company targeting unmet medical needs in serious ocular and rare diseases. Adverum is advancing the clinical development of its novel gene therapy candidate, ADVM-022, as a one-time, intravitreal injection for the treatment of patients with wet age-related macular degeneration and diabetic macular edema. For more information, please visit www.adverum.com.
Forward-looking Statements
Statements contained in this press release regarding the events or results that may occur in the future are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include but are not limited to statements regarding the potential for ADVM-022 in treating wet AMD and DME. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include risks inherent to, without limitation: Adverum’s novel technology, which makes it difficult to predict the time and cost of product candidate development and obtaining regulatory approval; the results of early clinical trials not always being predictive of future results; and the potential for future complications or side effects in connection with use of ADVM-022. Risks and uncertainties facing Adverum are described more fully in Adverum’s Annual Report on Form 10-K for the year ended December 31, 2020 and any subsequent filings with the SEC under the heading “Risk Factors.” All forward-looking statements contained in this press release speak only as of the date on which they were made. Adverum undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Investor Relations Contacts
Myesha Lacy
Adverum Biotechnologies, Inc.
T: 650-649-1257
E: mlacy@adverum.com
Amy Figueroa
Adverum Biotechnologies, Inc.
T: 650-823-2704
E: afigueroa@adverum.com
Media Contact
Andrea Cohen
Sam Brown Inc.
T: 917-209-7163
E: andreacohen@sambrown.com
AC – Inflammation was Mild and Decreasing over Time in Cohort 3
AC – Inflammation was Mild and Decreasing over Time in Cohort 3
VC – Inflammation was Mild and Decreasing over Time in Cohort 3
VC – Inflammation was Mild and Decreasing over Time in Cohort 3
Steroid Eye Drops Post Prophylaxis Decrease over Time in Cohort 3
Steroid Eye Drops Post Prophylaxis Decrease over Time in Cohort 3
Majority of Patients are Supplemental Injection Free after a Single IVT Injection of ADVM-022 in OPTIC
Majority of Patients are Supplemental Injection Free after a Single IVT Injection of ADVM-022 in OPTIC
Robust Aflibercept Expression Levels Observed for Both Doses in OPTIC
Robust Aflibercept Expression Levels Observed for Both Doses in OPTIC; Within Modeled Therapeutic Range, Reaching Top of Dose Response Curve
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.