Unraveling stress granules: key to understanding neurodegenerative diseases
GA, UNITED STATES, March 3, 2025 /EINPresswire.com/ -- A comprehensive review provides new insights into the pivotal role of stress granules (SGs) in cellular stress responses and their profound implications for neurodegenerative diseases. It discusses how these dynamic, membraneless organelles interact with other cellular components, offering a deeper understanding of their contribution to conditions such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). These findings open the door to novel therapeutic approaches that could help slow disease progression and enhance our knowledge of cellular stress management.
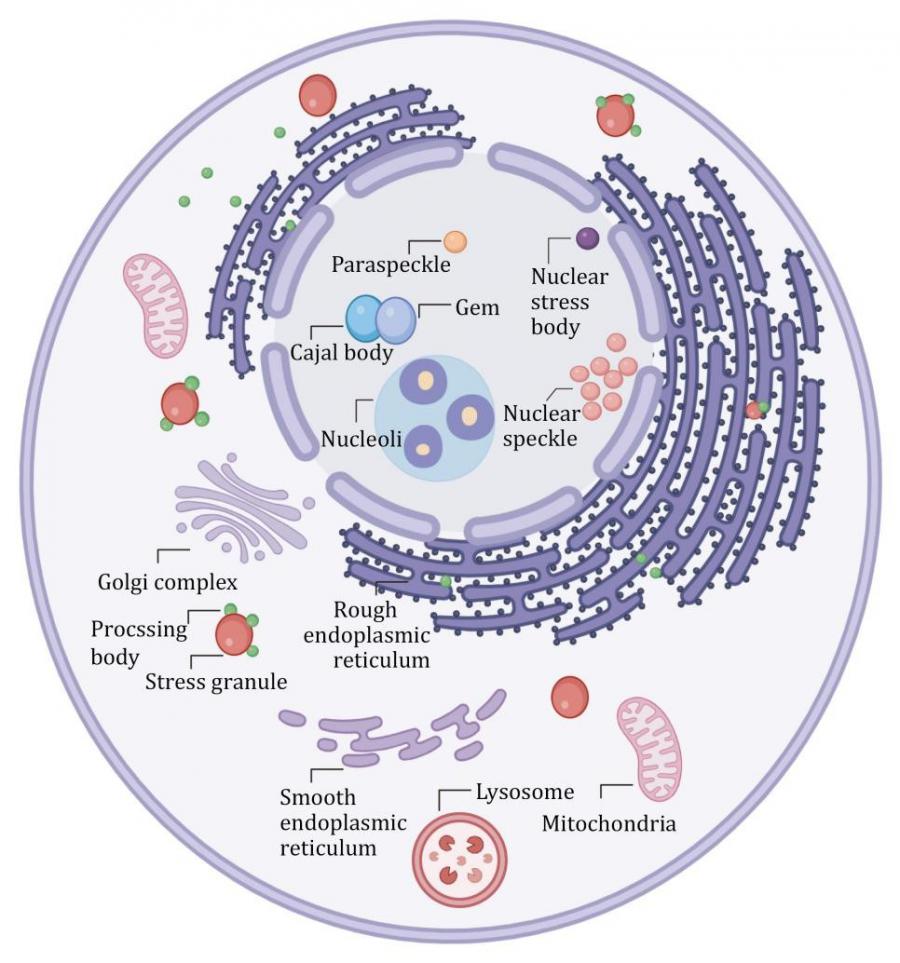
Stress granules (SGs) are transient organelles that form when cells experience stress, serving as critical hubs for RNA metabolism and cellular survival. Comprised of RNA-binding proteins and nucleic acids, SGs exhibit liquid-like properties and are involved in processes like mRNA regulation and protein synthesis. However, the dysfunction of SGs has been implicated in several neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). While their role in pathology has been hypothesized, the exact mechanisms by which SGs interact with other cellular structures and contribute to disease progression, particularly through interactions with other organelles, remain elusive. This knowledge gap has driven a need for in-depth research into SG dynamics and their cellular interactions.
In a review (DOI: 10.1093/procel/pwae057) published in Protein & Cell on October 23, 2024, researchers from Peking University Health Science Center discusses how SGs interact with various cellular organelles and how these interactions influence neurodegenerative disease pathways. Using cutting-edge techniques such as proximity labeling and biochemical fractionation, critical shared components between SGs and other cellular structures have been identified, including processing bodies, paraspeckles, and membrane-bound entities like lysosomes and the endoplasmic reticulum. These findings offer fresh insights into the interactions between SGs and other cellular organelles, and their collective roles in RNA metabolism and cellular responses to stress.
One of the key discoveries is the dynamic relationship between SGs and promyelocytic leukemia (PML) nuclear bodies, which plays a crucial role in the clearance of toxic intranuclear inclusions linked to neurodegenerative diseases. The review also sheds light on the role of Annexin A11 in facilitating the interaction between SGs and lysosomes, thus influencing the transport and stability of RNA granules. Furthermore, the review highlights the potential of SGs as biomarkers for early diagnosis and monitoring of ALS and FTD, signaling the promise of non-invasive diagnostic tools in the future.
Dr. Peipei Zhang, the corresponding author of the review article, emphasizes the significance of understanding SG dynamics: This review discusses how SGs coordinate with other organelles to regulate cellular stress responses. Dysregulation of these interactions may drive neurodegeneration, presenting new targets for therapeutic intervention.
The findings discussed in this review article hold considerable promise for advancing the development of targeted therapies for neurodegenerative diseases. By mapping the interactions between SGs and other cellular organelles, this review lays the foundation for therapeutic interventions aimed at modulating these processes to slow disease progression. In addition, the potential for SGs to serve as biomarkers for early-stage diagnosis could improve patient outcomes by enabling timely intervention. This review underscores the importance of further investigating cellular organelles to identify novel strategies for combating neurodegenerative diseases, potentially reshaping the future of disease management and patient care.
DOI
10.1093/procel/pwae057
Original Source URL
https://doi.org/10.1093/procel/pwae057
Funding information
This work was supported by the National Key Research and Development Project of China (2023YFC3505000 to P.Z.) and the Beijing Natural Science Foundation of China (7244365 to P.Z.).
Lucy Wang
BioDesign Research
email us here
Distribution channels: Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release